Treatments applied to the surface after a product is cast or assembled can radically alter its properties. This is true of many construction and manufacturing materials but especially metals.

Most early methods were directed at adding corrosion resistance; galvanisation was first used in 17th-century India to prevent armour rusting, and chromic acid anodising was first used on British seaplanes in 1923. While corrosion protection remains an important motivation, today’s surface treatments are often applied to adjust conductivity, insulation, electrical resistance, lubrication, adherence, porosity, ductility, tensile strength, hardness, softness, temperature resistance or simply appearance.
What Is Anodising?
Anodising is an electrolytic process that produces structured and powerfully bonded metal oxides on aluminium, titanium, magnesium and other alloys. Oxide layers can be as thin as ½ micrometre or as thick as 100, meshed into a restructured sub-layer.
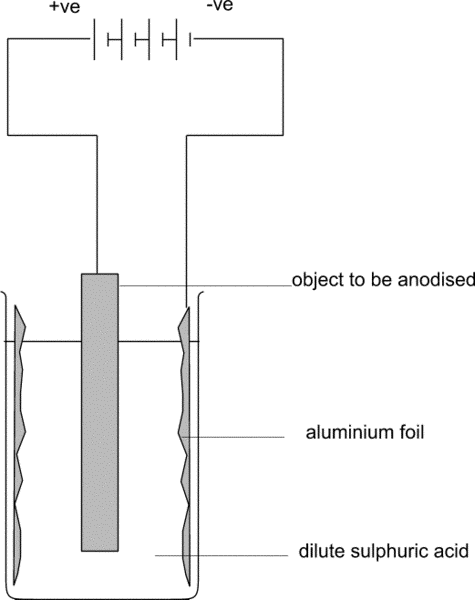
Although often complex in execution, the basic principle is simple. The treated surface is the electrolytic anode in a dilute acid solution – hence the name. Surface pre-treatments, choice of acids and their concentration and the currents applied all influence the result. Often several variations are applied in sequence. Monitoring results non-destructively is also vital during these surface treatments.
Other chemicals can be added during anodising or take advantage of the porosity achieved to penetrate it afterwards. For example, adding dyes enables anodised products to have almost any colour imaginable. Other post-treatments include sealants, lubricants, catalysts and sacrificial chemicals that provide self-healing properties.
Anodising is a common pre-treatment to improve bonding for other surface treatments like paint, polymers, adhesives or concrete.
Today, anodized aluminium is seen in a vast range of products, from small items like keys, iPods, mobile phones, electrolytic capacitors, cameras and cookware up to larger items like building facades, roofs, doors and window frames, street lights, firearms and sports equipment.
How It Is Done
Drilling and other production processes must usually be completed first. Surfaces then need careful preparation. Polishing can be achieved mechanically, chemically or electrolytically.
Anodising then proceeds in several stages using different acids at different concentrations and temperatures. Sulphuric, oxalic, chromic and phosphoric acids are the most common, usually at 10-15% concentrations and temperatures between 20 and 45 degrees Centigrade. Higher acid concentrations, lower temperatures and high voltages can produce harder, less porous surfaces.
Anodising is often the cheapest and most effective way of finishing products or improving existing surfaces.